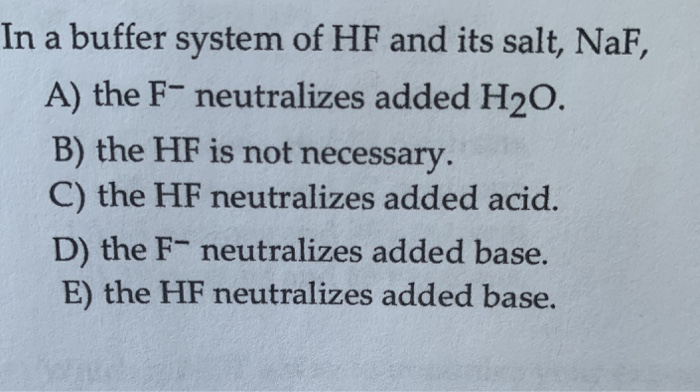
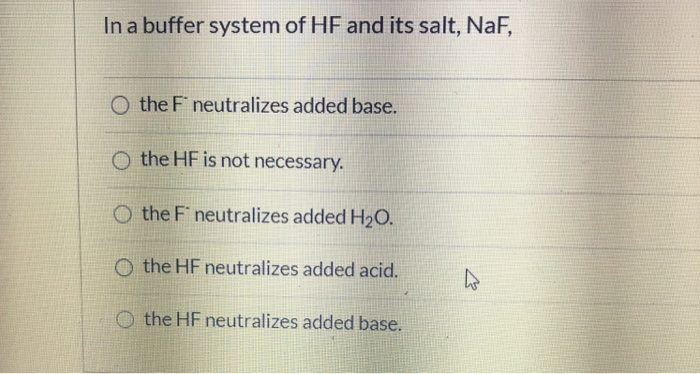
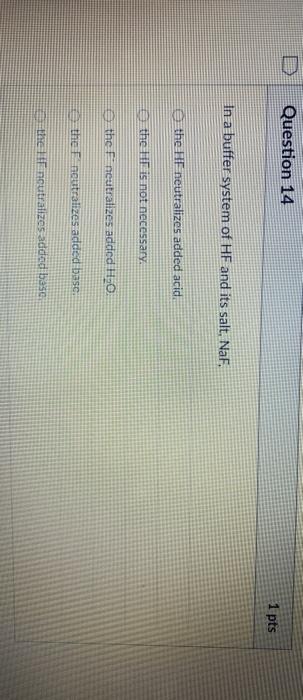
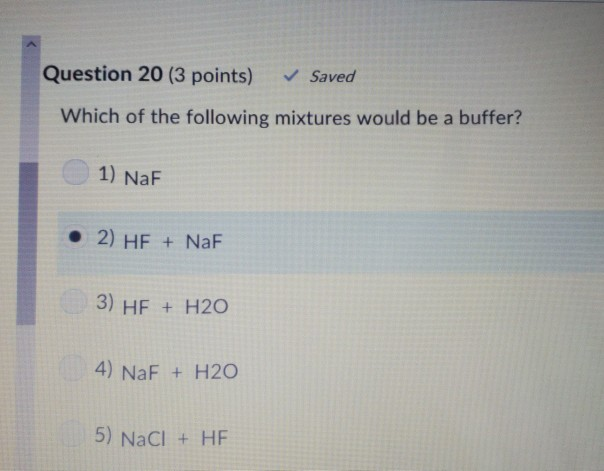
In a Buffer System of Hf and Its Salt Naf
NaF aq H2O l HF aq NaOH aq Question. 02103 g KCl100 mL c.
3 18 mM Na.
. How many moles of water are made from complete reaction of 22 moles of oxygen gas with. Check out a sample. Which of the following equilibria could be used to support the claim that the addition of a small amount of NaOH to the buffer will result in only a very small change in pH.
A mixture of a weak acid and its conjugate base or a mixture of a weak base and its conjugate acid is called a buffer solution or a bufferBuffer solutions resist a change in pH when small amounts of a strong acid or a strong base are added A solution of acetic acid and sodium acetate CH 3 COOH CH 3 COONa is an example of a buffer that consists of a weak acid and. B Aniline C6H5NH2 has a pKb of 940. If 020 mol of.
The usual approximation yields. Bicarbonatecarbonate buffer solution 17 mM NaHCO. Cells were collected and lysed in lysis buffer containing 20 mM HEPES pH 74 50 mM NaCl 50 mM NaF 10 mM β-glycerophosphate 2 mM EDTA and 03 CHAPS.
It can be prepared by combining a weak acid with a salt of its conjugate base. Dissolve 05712 g NaHCO. We therefore expand the.
Kc 15 1011. Dissolve salt in filtered deionized water a. Calculate the pH of the solution after the addition of 500 mL of 10 M HCl.
Clarified cell lysates were incubated with. The Ka of the weak acid HA is 20 x 10-5. Because HF is a weak acid the equilibrium strongly favors the right side.
2562 g of salt was quantitatively converted to free acid by the reaction 30 ml of 02 M H2SO4 the barium. 02210 g NaF100 mL b. It can be prepared by combining a weak base with its conjugate acid.
Your pH should go to two places past the decimal point ie. It can be prepared by combining a weak base with its conjugate acid. And 07631 g Na.
It can be prepared by combining a weak acid with a salt of its conjugate base. Changjun Wu Yu Wu Xiaodong Xu Dongsheng Ren Yalun Li Runze Chang Tao Deng Xuning Feng and. A 100 L buffer solution is 0280 M in HF and 0280 M in NaF.
Chemistry of life lab 3 answers. Want to see the full answer. HF K a 67E 4 Solution.
The aerial sections were collected and grounded with a plastic pestle followed by solubilization in 40 µl of SDS buffer 3 wv SDS 30 mM Tris-HCl pH 80 10 mM EDTA 10 mM NaF 30 wv. It can be prepared by combining a strong acid with a salt of its conjugate base. Consider a solution initially containing 040 mol fluoride anion and 030 mol of hydrogen fluoride HF.
What is the pH of a solution which is 012 M in aniline and 038 M in anilinium ion. A buffer is a solution that will resist a change in pH as the weak acid can react with added base and the conjugate base can react with added acid. The major equilibria in the buffer system are represented above.
ACS Applied Materials Interfaces 2022 14 8 10467-10477 Energy Environmental and Catalysis Applications Publication Date Web. Synergistic Dual-Salt Electrolyte for Safe and High-Voltage LiNi 08 Co 01 Mn 01 O 2 Graphite Pouch Cells. It can be prepared by combining a strong acid with a salt of its conjugate base.
Want to see the full answer. Consider a solution initially containing 040 mol fluoride anion and 030 mol of hydrogen fluoride HF. In 4 L filtered deionized water 5.
Find the pH of a 015 M solution of NaF. The pH of a buffer solution. The pH of a buffer solution.
Check out a sample QA here. If the reaction below begins with 0150 M sodium fluoride what would be the concentration of NaOH at equilibrium. The Ka for HF is 68 x 10-4.
Calibration stock solutions 1 mgmL as the anion. But on calculating xC a 01 015 07 we find that this does not meet the 5 rule for the validity of the approximation. How many moles of water are needed to react with 22 moles of Li2OLi2O.
The molecular mass of an organic acid was determined by the study of its barium salt. A Calculate the pH of an aqueous solution of the salt NaA which was made to a concentration of 0080 M NaA. Ka 66 x 10-4 The equation above represents the acid.
What is a buffer. If 020 mol of. Academiaedu is a platform for academics to share research papers.
The reaction is F- H 2 O HF OH. NaF aq H2O l HF aq NaOH aq Expert Solution.

Buffers And Acid Base Titration Common Ion Suppose We Have A Solution Containing Hydrofluoric Acid Hf And Its Salt Sodium Fluoride Naf Major Species Ppt Download

Solved In A Buffer System Of Hf And Its Salt Naf A The Chegg Com

Chemistry 5 9 Buffers Flashcards Quizlet

Oneclass Consider The Buffer System Of Hydrofluoric Acid Hf And Its Salt Naf Hf Aq H2o L A œh3o
Solved In A Buffer System Of Hf And Its Salt Naf O The F Chegg Com
Solved Question 14 1 Pts In A Buffer System Of Hf And Its Chegg Com
Solved Question 20 3 Points Saved Which Of The Following Chegg Com

Solved 16 Consider The Buffer System Of Hydrofluoric Acid Chegg Com
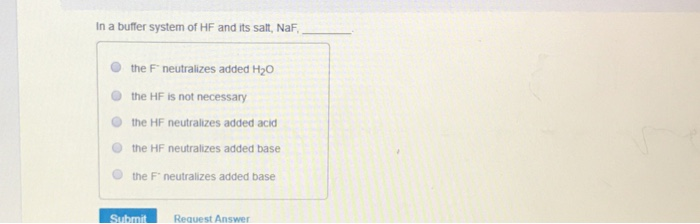
Solved In A Buffer System Of Hf And Its Salt Naf The F Chegg Com

Chemistry 5 9 Buffers Flashcards Quizlet
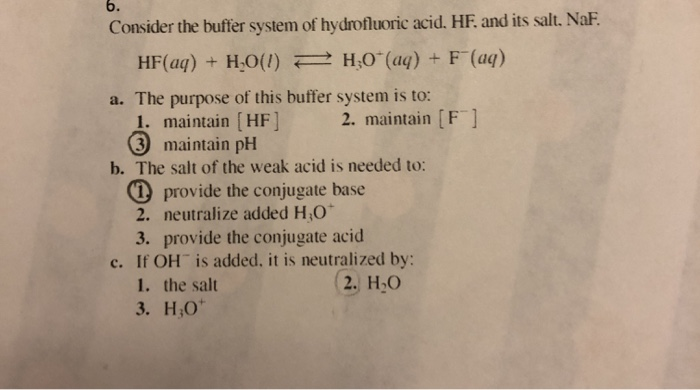
Solved Consider The Buffer System Of Hydrofluoric Acid Hf Chegg Com

Which Mixture Will Form A Buffer Youtube
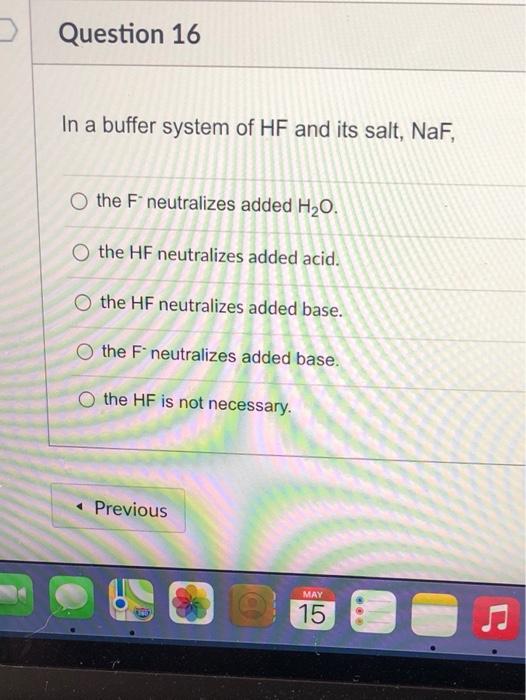
Solved Question 16 In A Buffer System Of Hf And Its Salt Chegg Com

Solved 41 In A Buffer System Ohf And Its Salt Naf A The Chegg Com

Example Ph Of A Buffer Hf Naf Youtube

Solved The Addition Of Hf And To Water Produces Buffer Sclution Nabr Hbr Kno3 Koh Nacl

Which Mixtures Form Buffer Solution P 100 Ml Of 0 200 M Hf 200 Ml Of 0 200 M Naf Youtube
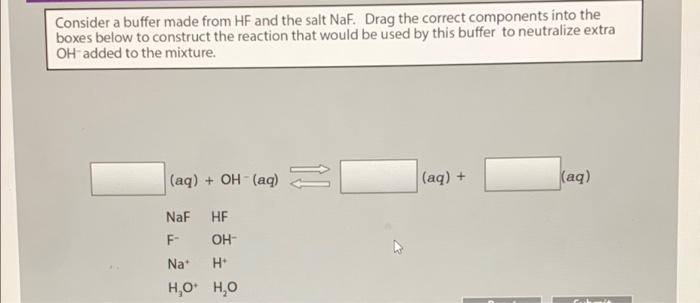


Comments
Post a Comment